Solar panel installation has increased tremendously in homes in the past few years, with about 40% more homeowners converting to solar energy in the last 5-10 years.
A primary reason for the trend is the desire to move to clean, sustainable, and renewable energy sources. However, after making the switch, homeowners can also enjoy additional perks, such as tax credits, rebates, and a reduction in their energy bills.
9 Advantages of Solar Energy
- Renewable and Sustainable
- No Harmful Gases or Smoke
- Quiet Operation
- Low Maintenance
- Sunlight Is Free
- Flexible Configurations
- Evolving Technology
- Practical in Rural Areas
- Rebates and Tax Incentives
4 Disadvantages of Solar Energy
- Expensive Start-up Costs
- Bad Weather
- Requires Space
- Can Be Limited by Surroundings
There is a lot to consider before making your home one that's powered by the sun, such as overall costs, finding the right installer, and how solar energy can affect your home.
What Is Solar Energy?
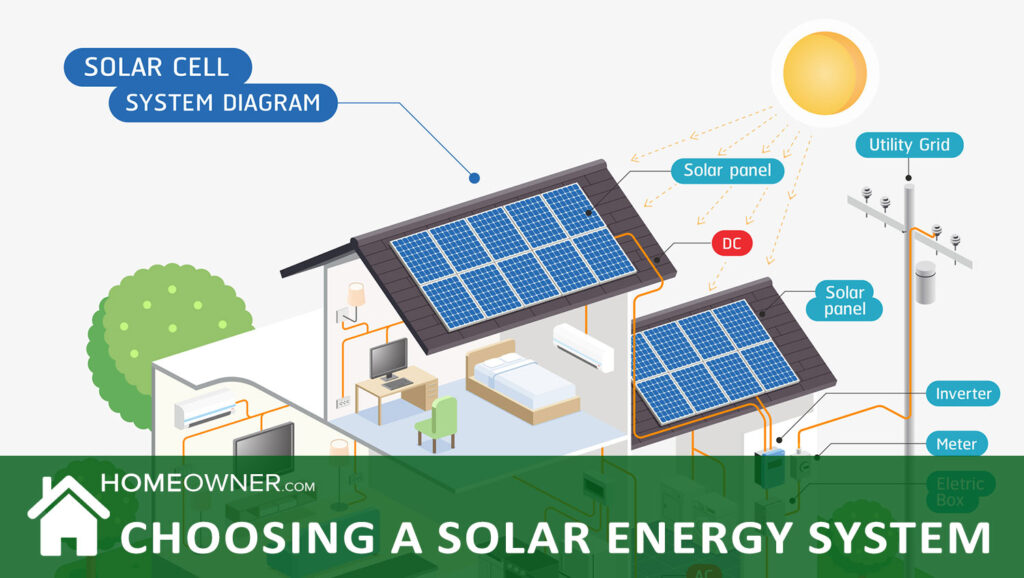
Before choosing a solar system for your home, it helps to understand how solar works. Solar energy is natural energy derived from the sun. To create solar energy, special equipment using solar photovoltaic (PV) technology absorbs sunlight and converts it into energy. One of the most common types of equipment of this kind is a solar panel, which buildings can place on their roofs to collect sunlight for energy.
This energy then transforms into electricity, which people can use to power their TVs, appliances, and more. Extra energy produced by solar equipment can be stored until it's needed with the help of battery storage, so excess energy won't go to waste.
Since the sun will always produce sunlight, solar energy is one of the most sustainable forms of energy. Also, the equipment used to convert sunlight into solar energy does not produce the same environmental hazards as other energy conversion processes, so it's also a cleaner form of energy.
What Does “Going Solar” Mean?
The "going solar" trend is one that's become more prominent as environmental advocates have pushed for the need for clean energy. This has been the biggest year for growth in the residential solar power industry, with a record-breaking 30% increase in installations from the previous year.
When people talk about "going solar," they typically mean that they've switched their home to be mostly or entirely powered by sunlight. Homeowners who have gone solar have likely added solar panels to their roofs or open land to convert sunlight to electricity.
Going solar can also refer to replacing typical electric or gas heating with solar heating. Solar heating again uses the sun's energy but to heat air rather than power a home, resulting in solar heat.
Advantages of Solar Energy
There are many advantages of solar energy. You can compare the following solar power advantages and disadvantages to help you decide if solar energy is right for your home.
Renewable and Sustainable
Solar energy is a renewable form of energy and thus is sustainable energy. This means that the sun will come up pretty consistently forever, so you can rely on this power always being there. Sure there will be nights and cloudy days, but sooner or later, the sun will shine again and this "free form of renewable energy" will be back for us to use. Oil is not a renewable energy source, which means that once it's used, it's gone forever (it's in limited supply).
No Harmful Gases or Smoke
More benefits of solar energy include the fact that solar energy creates no immediate pollution. While burning oil releases harmful greenhouse gases, carcinogens and carbon dioxide into the air, solar runs cleanly, converting sunlight to usable energy without the combustion and smoke.
Quiet Operation
Another of the advantages of solar energy is that solar power is pretty much a quiet process. You can harness as much solar energy from the sun as you want and there is no resulting noise pollution. This is pretty rare in the world of "energy making" power sources like noisy gas powered generators. If you appreciate peace and quiet you'll definitely agree that this is one of the best solar power advantages.
Low Maintenance
Very little maintenance is needed with solar panels. It helps to know some solar maintenance tips, but usually an occasional cleaning is all you'll have to do, with a more in-depth inspection every once a year. This is because solar panels don't really have any moving parts and therefore there is less friction, wear out or breakage to worry about. One of the nicer benefits of solar energy.
Sunlight Is Free

Energy from the sun costs nothing, making solar power cheaper than grid electricity. This is definitely one of the advantages of solar power because, once you purchase and set up the solar equipment to collect and convert energy from the sun, it will run everyday and you won't have to pay over and over for your power. Although there is an initial investment involved, the payoff can be very worth it, when you eliminate your monthly utility bills long term.
Flexible Configurations
Solar power is also very flexible. You can have different solar arrays in different areas without having to run wiring from one to the other. For example: You can put one solar array on your roof to generate power for your home, and also have another smaller one near your garden to power backyard lights or anything else outside that typically needs less electricity.
Evolving Technology
The advantages of solar power over fossil fuels are many, including the fact that the technology of solar power generation is only advancing with time. Odds are, solar power will become even more affordable and practical in the future, while oil and gas burning remains rather ecologically primitive and clearly limited in supply.
Practical in Rural Areas
The advantages of solar energy use in remote areas are also big. Unlike fossil fuel sources, solar power is very practical for remote areas. The cost to install solar power in areas such as this would be far less than the cost to run power lines.
Rebates and Tax Incentives
Another one of the benefits of solar energy is that many regions are now offering rebates for installing solar power in residential homes. This helps bring the cost of solar power down, while encouraging more families to make the move toward sustainable energy.
Disadvantages of Solar Power
While there are obviously many advantages to solar power, there are also some disadvantages of solar energy that you should know about. By weighing the pros and cons of solar energy, you can better decide if solar power is right for you.
Expensive Start-up Costs
One solar power disadvantage is that although it's sustainable once set up, getting started with solar power can be confusing and expensive. Solar panels can cost a lot of money and solar installation isn't cheap. Fortunately, websites like ours exist to make this whole process much more affordable and easy to understand.
Bad Weather
Also in most parts of the world, there will be those periods of cloudy weather and rain, so you will still have some limitations in regards to making the energy and you will most likely have to rely on gas and oil again at some point. However using a battery bank to store power so you can use it later greatly reduces and can even completely eliminate this drawback.
Requires Space
A solar energy installation usually requires some space in order for the solar system to be efficient in producing electricity. This can be a disadvantage in areas where space is short or expensive such as inner cities. However if you have a large roof, you can make more power and this isn't an issue.
Can Be Limited by Surroundings
Another one of the major disadvantages of solar energy is that you are sometimes limited by your surroundings. Tall houses, trees and buildings can really put a damper on your solar system's performance and potential by blocking sunlight from getting to your panels.
In the end, the disadvantages to setting up and maintaining a solar power system are far less than the advantages of solar energy and the overall benefits gained by both people and the environment.
How Solar Panels Work

Solar panels work by collecting solar energy from the sun. How well they work depends on how much light energy they get via the solar cells.
Solar cells are typically combined to make solar panels, otherwise known as PV panels, solar modules, or PV modules. One or more of these panels grouped together constitutes a PV array or solar array.
Solar panels are used to convert solar photovoltaic energy into DC (direct current) electricity. A power inverter is then used to convert that DC electricity to AC (alternating current) electricity, which is what we use in our homes.
How Solar Works In Your Home
Your solar panels gather energy from the sun and send it to your photovoltaic components (inverter), which convert it to AC power. Then, the AC power enters your home through the utility panel and is distributed to appliances or lights in the house.
If you don't go through your breaker panel, you can also access the power by plugging appliances directly into the inverter's power sockets.
However, if your system is connected through the utility panel, all the excess electricity can be exported back into the utility grid and credited to your electric account. If you used a large enough solar energy system, you could make your utility meter spin backwards.
This is how solar energy works in your favor when you hook up to the grid and actually make money (in the form of an energy credit) from your utility company.
How Much Money Can I Save With Solar?
Converting your home to solar energy can significantly reduce the amount you spend with each energy bill. However, the amount you save ultimately depends on the amount your solar panel system produces. In other words, the more sunlight your panels can convert, the more you're likely to save, as you'll use less and less electricity as your solar usage rises.
Homes with lots of square footage on their roofs that are in ideal spots to absorb plenty of sunlight can produce optimal solar energy. However, smaller homes near wooded areas that don't get a lot of sunlight may not be able to produce as much energy and will still need to rely largely on electricity. These differences can affect your savings.
According to estimates, the average household can save about 90% on their energy bills by going solar.
Homeowners should consider whether their solar energy system will be covered under a home warranty or homeowners insurance plan to determine if their savings from solar panels are worth it. These policies can protect high-cost parts of a home.
Many home insurance policies will cover solar panels, but your premiums could rise as a result. Home warranties usually don't cover solar panels, so you could be on your own for repairing them should your insurance policy not provide adequate protection. Check with a representative about how going solar could affect your policies.
What Is Net Metering?
Net metering allows energy from solar energy systems to go back into the grid if it's not used. This not only helps other customers have enough energy to power their homes, but it can also save solar energy customers money for their extra energy production from PV panels.
To make net metering work, customers get billed only for their net energy usage, which is the amount they've produced versus what they've used. In states that enact bill credits for net metering, the customer receives a credit on their bill for all unused electricity generation.
How Net Metering Programs Work in Different States
Most states require that customers receive an energy bill credit for unused solar energy that is recycled back into the grid. New Jersey, California, Florida, and Ohio are among the states with mandatory net metering rules.
Currently, only three states – Alabama, South Dakota, and Tennessee – do not provide incentives for net metering.
Each state's policy differs, but most have caps on how much excess energy resources you can produce. For example, Nevada does not allow net metering if a customer can produce more energy than 100% of the amount it uses.
Check with your state's public utilities commission to learn more about its net metering incentives.
How Much Do Solar Panels Cost?
Solar panels come in all different shapes and sizes. There are many widely varying solar panel prices. When buying solar panels, choose panels with a good balance between power and price. Sometimes, the cheapest solar panels are not always the best ones for you.
Factors That Affect The Price Of Solar Panels
Solar panel pricing can be a little tricky. You must take into account factors such as durability, average lifespan, warranty, efficiency, and power producing capability before you can really judge if a solar panel is priced too high or not. Always judge the cost of solar panels based on the quality and power capacity of the solar panel – not just the lowest price.
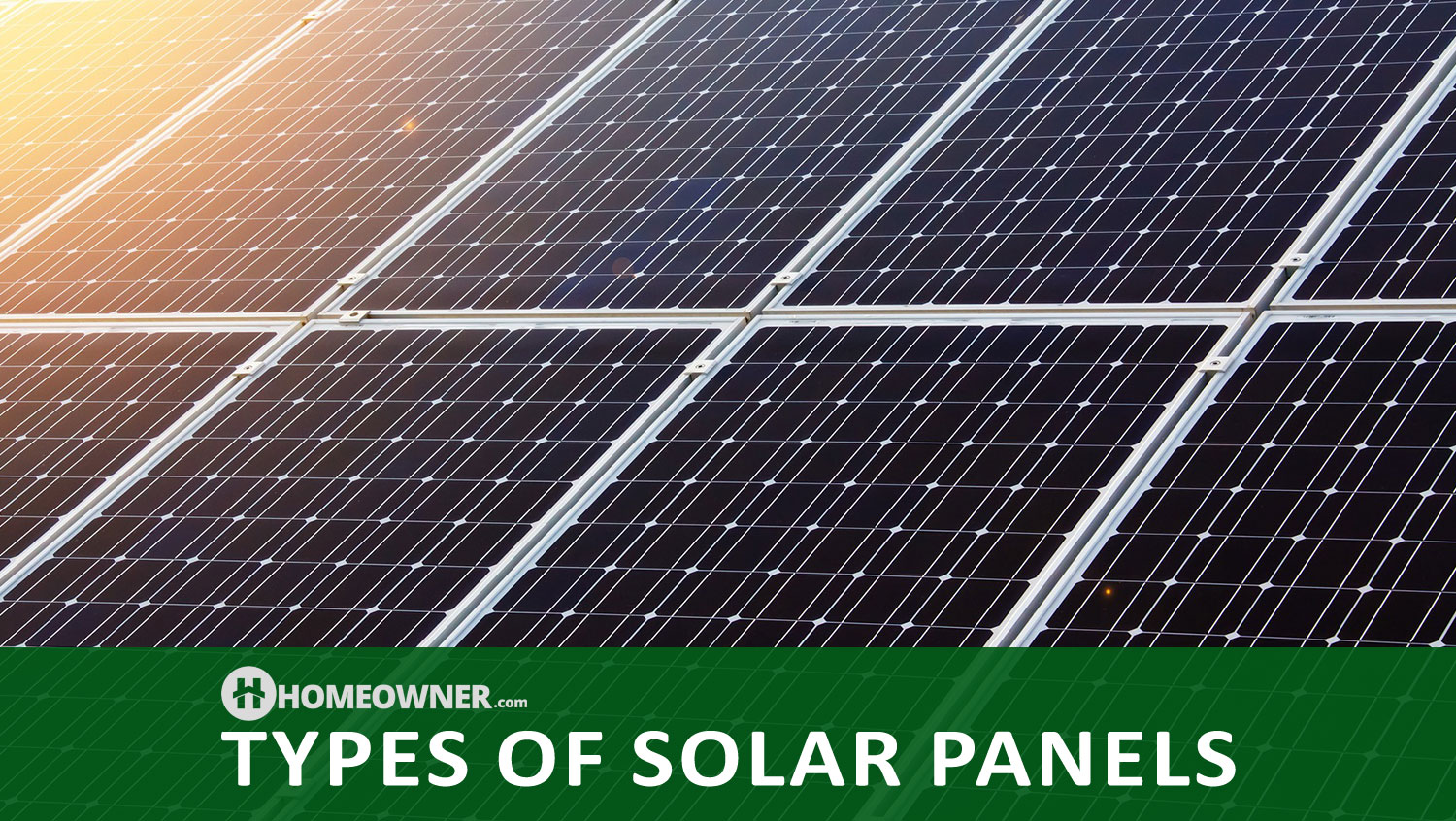
Types of Solar Panels and Price Per Watt
There are three types of solar panels and these include single crystal (mono-crystalline), poly-crystal (multi-crystalline), and amorphous silicon.
Single crystal solar panels are currently the most efficient type available, meaning that they produce the most power per square foot of module. This makes them one of the most expensive at a price of about $4-$6 per watt average. The cost of solar panels like this is high, but since they produce more power, it could very well be worth it.
Poly crystal solar panels are slightly less efficient but cost a little less at a price of about $3-$5 per watt average. The cost of solar panels like this is typically lower, but you'll lose some efficiency.
Amorphous silicon solar panels (thin film solar panels) are less efficient, require more space, but are more flexible and can be mounted easily on roofing tiles or shingles. These are the least expensive solar panels available at a price of about $2-$4 per watt average. The cost of solar panels like this would be the lowest, but you'll lose even more efficiency and have to use more of them.
General Solar Panel Retail Costs
Solar panels for sale in stores and online are typically priced according to their watts. Below we provide a general guideline of what you would expect to pay for good quality multi-crystalline solar panels.
- A 15 watt solar panel would typically have a price of: $50 - $100
- A 40 watt solar panel would typically have a price of: $200 - $300
- A 100 watt solar panel would typically have a price of: $300 - $500
- A 200 watt solar panel would typically have a price of: $500-$900
The more powerful you want your solar panels to be, the more you will pay. If you build your own solar panels, you can save a lot of money and bring your solar panel cost down.
Please note that the cost of solar panels is constantly changing with the steady advancement of solar technology thus solar panel price is subject to fluctuation.
Where Can I Find a Solar Installer?
Solar installers are usually easier to find in larger areas or locations that prioritize clean energy, like solar electricity. However, it's not impossible to find a solar energy installer even in more rural areas.
First, ask for solar installer recommendations from people you know. You might have friends and family who have already had a good experience with a company they'd highly recommend.
Your state or local government website can also be a helpful resource. Look for links relating to its energy or utility commission for directories or help with finding a legitimate solar installer.
You could also talk to other home services professionals you've used for recommendations. These professionals are often well-connected with one another in an area, so plumbers, electricians, or roofers might know of solar installers in the area.
Tips for Finding a Quality Solar Installer

Once you have a few potential solar installers on your list, consider the following tips to narrow your options and find the best solar company for your home.
Read Reviews
One of the best ways to get an idea of a company's reputation is by reading its reviews online. A few places to do this include:
- The company's website (testimonials)
- Better Business Bureau (BBB)
- Google business reviews
- The company's Facebook page
- Customer review websites, such as Yelp, Consumer Affairs, and TrustPilot
- Local commerce websites
- Reddit or Nextdoor pages for your area
- Local Facebook groups
Hyperlocal companies and individual installers may not have as many reviews as larger businesses, but you can still usually find some comments floating around. If not, take to your own social media pages to ask your friends and family if they've had any experience with the company.
Research Past Work
Before contracting with a solar installer for your new power system, find out what you can about the installer's past work. Some service providers are very transparent, posting photos and customer testimonials on their Facebook pages or websites. This makes it easy for you to see what you can expect from the installer.
Others require a little more digging. You may be able to find what you need in past reviews. You can also contact the company to ask for photos of past jobs.
Research Services
Check out the solar installer's website to learn more about its services and how it performs them. Some solar installers focus only on specific solar services, such as solar water heating or commercial solar power systems. Getting an idea of the services they offer ahead of time can narrow your options to find an installer that's a good fit for what you need.
Ask for (and Compare) Free Estimates
Many solar installers will visit your property to give free estimates on the cost of your new solar energy system. Take advantage of these offers, as they allow you to compare one company with another. Consider the solar energy technology and equipment, labor charges, fees, and installation time when comparing each estimate.
How Much Do Solar Installers Charge?
Solar technology comes in different forms to fit various homes and electricity production needs. Your solar installer's quote for solar energy systems could vary significantly from other homes. Prices also vary by state and the current supply and demand for solar energy technology.
The average United States household pays about $15,000 for their solar energy systems after tax incentives. Prices increase with higher wattages. For instance, an 8 kW system costs thousands of dollars more than a 3 kW system.
Consider getting a few quotes to compare equipment and pricing. Then, check your state's solar production incentives for homeowners to better understand what you can expect to pay for solar installations.
Solar Energy Tax Credits
When you convert your home to solar energy, not only can you save on your energy bills, but you can also qualify for solar energy tax credits. The federal government gives homeowners who have installed a solar photovoltaic system a percentage back in the form of a tax credit. Some states also have solar energy incentives for homeowners to get breaks on their bills.
The federal tax credit is for residential taxpayers only. Homeowners must install the solar system and begin generating solar energy during the same tax year for which they're claiming the credit.
For example, let's say the federal government gives taxpayers a 26% credit for installing a solar system. Therefore, if you paid $15,000 for your system, you can claim $3,900 in a tax credit. Keep in mind that federal tax credits change from year to year.
Examples of State Solar Energy Incentives
Each state can decide what tax credits or other incentives it wants to give homeowners to promote energy efficiency through solar technology.
For instance, Kansas offers a renewable energy property tax exemption for any renewable energy equipment installed on a property for up to 10 years if the homeowner filed for the exemption after 2016. NV Energy customers in Nevada who have installed solar energy systems can receive rebates on their solar energy production, as much as $0.0550 / kWh.
DIY Solar: Should I Install My Own Solar Panels?
A DIY solar installation can be tempting because it could fit within your budget better than hiring a professional to do it. In fact, you can build your own solar panels and using helpful DIY kits.
Keep in mind, DIY solar is not for everyone. Choosing a professional installer rather than doing it yourself can keep your solar technology protected under warranty. Many installers have warranties that cover labor and equipment for a specific amount of time to protect your purchase.
Solar Powered Homes
There are many advantages to installing solar in your home. Today, almost 3% of American homes are powered by solar, and this number is growing. Solar energy is clean, sustainable, and renewable – plus, you can enjoy additional perks such as tax credits, rebates, and tremendous energy savings.
Solar energy is becoming more common every day in homes and businesses. Solar home installations are more affordable than ever before as the trend catches on and more households convert to solar energy. Contact us today for a free estimate on your solar home installation.